How to pH Test Soap the Right Way & Why It Matters!
One of the common questions that come up on Modern Soapmaking, especially in hot process tutorials and liquid soapmaking tutorials, is how to pH test handmade soap. It's easy to understand why when there is so much conflicting information on the internet pH testing soap!

I hope to clear up a lot of common misconceptions about the why and how of pH testing handmade soap in this article, and will be including information and examples for various kinds of soap! Before we dive in, let's review...
What is pH anyhow?
pH is a measurement of the hydrogen ion concentration in an aqueous (water) solution. Solutions with a high concentration of hydrogen ions have a low pH, while solutions with a low concentrations of hydrogen ions have a high pH.
Neutral is a pH of 7, while anything above that is alkaline, and anything below is acidic. Most people are familiar with the pH of household substances like citrus juices, milk, and various cleaning products, but just in case you aren't, here's a quick approximate comparison chart of common materials on a pH scale:

pH testing handmade soap typically shows a result between 8 and 10, but can creep up near a pH of 11.
As far as I'm aware (and have been able to find scientific evidence for - the important part), it is impossible for a handmade soap to fall near neutral or below without using an emulsifier to keep the soap molecules within the solution. I know that soapmakers frequently state that they can or do create neutral pH soap, but such products don't tend to be true soap, meaning that they aren't composed mainly of the alkali salts of fatty acids.
Sometime ago, Kevin Dunn asked the handmade soapmaking community to please send him a soap with a pH of 7 or below, and as far as I'm aware, no one was able to do so.
Important Notes about pH Testing Soap
There are two key points I want to jump into here, before we start talking about pH testing soaps.
First, it's important to keep in mind that pH is a measurement of hydrogen ions in an aqueous solution. It is not a measurement of hydrogen ions in whatever substance you feel like measuring! Obviously, when you are looking at bar soap, it's a relatively solid material - not an aqueous solution. In order to properly test the pH of a bar soap, you should attempt to use a solution. (We'll get more into that later on.)
The second important note is that pH is not a direct relationship to how harsh or mild a product is (whatever that means in the first place!) When it comes to skin chemistry, a common misconception is that your skin has a pH - it doesn't, your acid mantle does. The acid mantle can have a varying pH between 4.5 and 6.2, depending on your ethnicity, age, and gender, as well as the area of the body, the humidity, and various other factors. Your acid mantle acts as a barrier on top of your skin that helps discourage the growth of fungi and bacteria.
In general, brief exposure to slightly acidic or slightly alkaline material (like using handmade soap, which is slightly alkaline) does not harm the acid mantle. Healthy skin can rebalance the acid mantle in a very short amount of time (in as little as 15 minutes to as long as 90 minutes). Long-term or prolonged exposure (such as applying a high pH product to your skin and leaving it on for hours) can cause damage, just as exposure to extremely acidic or extremely alkaline (like sodium hydroxide) material can.
Even if you wash your skin with tap water (typical pH between 6 and 8.5 depending on the source) and no soap or cleanser at all, the pH of your acid mantle will slightly increase immediately following washing. (In a study of infants, water-only washing sees a pH rise of 1.1 while alkaline soap, like handmade soap, sees a rise of 1.2 points.) Furthermore, there are plenty of high pH products on the market that are less irritable to the skin than lower pH products! (One study found that Johnson's Baby Oat soap, with a pH of 12.35, was the least irritating soap tested, while another soap with a pH of 9.36 was the most irritating.)
The recent onslaught of "pH-balanced" skin care products is largely a marketing ploy. So, pH should not be viewed as an indicator of whether or not a product is irritable to your skin unless it is being applied for hours at a time or has an extremely acidic or alkaline pH that could cause damage.
Well, what's the point of pH testing soap then?
A lot of soapmakers attempt to use pH testing soap as a indication of excess alkali, and this is flawed for a few reasons.
First, unless a soap contains a large amount of excess alkali, it could test within a "normal" pH range for handmade soap (between 8 and 10). And, if given enough time to cure, lye-heavy soap could eventually test normal as carbon dioxide works its magic on free alkali. (Fun fact: sodium hydroxide + carbon dioxide = sodium carbonate, which is soda ash!)
Plus, if you pH test handmade soap fresh out of the mold the soap could be unfinished. Saponification may not be fully complete. How quickly a properly made soap saponifies depends on the oils used (or the fatty acid profile of the formula), as well as the temperature of the soap and the environment, and can range from as little as 24 hours to multiple weeks.
The second reason using pH testing soap as a indication of excess alkali can be flawed is that soap can saponify unevenly. For instance, the middle of a batch of soap typically saponifies quicker, if gelled, while the exterior edges don't saponify as quickly. The top of a batch of soap could be slightly more alkaline, as the water (which carries the lye) evaporates out of the soap. Or in such cases of lye-heavy soaps, the lye solution or undissolved lye crystals can concentrate in pockets or specific areas in the final soap. Unless a soapmaker pH tests soap in just the right area, they could come out with a rating that ignores the presence of excess alkali.
Does this negate the usefulness of pH testing handmade soap? Yes and no!
It's much easier to identify lye-heaviness through visual cues, like the presence of liquid pockets or crystallization, rather than pH testing soap. If there is an unknown liquid or substances seeping out of a soap, a pH test can help you identify if the liquid is lye solution or separated oil, fragrance, or another additive. pH testing handmade your soap to confirm a suspicion when troubleshooting is the first step in knowing how to correct it.
Essentially, pH testing your soap show an abnormally high pH, then you know you need to either neutralize or continue cooking the soap (in the case of liquid or hot process soapmaking), or rebatch it (in the case of cold process soap). To neutralize or rebatch soap, you must first figure out how much excess alkali is present - either by reviewing your procedure (hence why it's important to check/calibrate your scale, detail your process, and use self-check measures to ensure accuracy) or titration to measure alkalinity. Scientific Soapmaking and Liquid Soapmaking are two books that detail the entire titration process!
Another way pH testing handmade soap can be useful is if you sell your soaps and want to adhere to good manufacturing processes. Conducting a pH test is one of many ways to conduct quality control, ensuring that your products fall within a specific range of "normal". However, pH testing your soap should not be used as the sole quality control measure, as it can be unreliable.
And finally, let's be realistic here, pH testing your soap satisfies curiosity and can be useful in understanding the chemistry behind saponification! If you ever get the urge to make multiple soaps with single oils, pH test them during the curing period every week to see how they change. Various saponified oils (and individual fatty acids) have differing pH levels, and the pH does tend to slightly drop over time.
The Do's and Don'ts of pH Testing Handmade Soap
If you do want to pH test your soap, it's important to start off on the right foot. When I was in the process of researching this article, I came across tons of blog posts and how-to videos out on the internet that gave instructions for pH testing handmade soap. Here's a rundown on some of the most common instructions I found:
Using Tongue Testing or Zap Testing to Check Soap Alkalinity
An extremely common recommendation I found as a method of checking the soap pH is to touch a bar of soap to your tongue. If touching the bar of soap to your tongue zaps similar to touching your tongue to a battery, it indicates a soap is lye heavy. Obviously, this doesn't actually indicate the pH level of a soap, it simply indicates the presence of free alkali.
I know plenty of soapmakers who use this method in their process, and while I won't argue its efficiency or efficacy, I will argue it's safety. If a new soapmaker comes across this advice and has not yet familiarized themselves with the appearance of lye heavy soap, tongue testing could result in serious injury.
 Just say "no" to tongue testing or zap testing!
Just say "no" to tongue testing or zap testing!
Additionally, if you are a soapmaker in business, tongue testing is a liability, safety, and GMP (good manufacturing practices) nightmare. GMP encourages robust testing and quality control among other processes, and while tongue testing accomplishes the job, it is unhygienic and dangerous. If an employee sustains an injury due to poor manufacturing practices, the liability falls on you, as the owner and creator of the procedures and manufacturing process. No, thanks! There are much better ways to pH test your soap.
Using pH Strips to Test Soap pH
The next most common method of pH testing soap I found online was using pH strips. Most of the tutorials and how-to instructions I came across were a variation of the following:
- Place water on the surface of the bar of soap.
- Rub the water onto the soap until it lathers.
- Dip the pH strip in the bubbles.
- Check the pH strip against the chart included.
 Using pH strips directly on a bar of soap can give you an inaccurate pH reading that is so far off you might think your soap is neutral!
Using pH strips directly on a bar of soap can give you an inaccurate pH reading that is so far off you might think your soap is neutral!
The first problem with pH strips is that the nature of soap interferes with the indicator dyes used to manufacture the strips, which can throw the reading off by several points. (In my testing, pH strips gave me a reading varying from a pH of 5 to a pH of 9 - a huge range!)
The second issue with this method of pH testing soap is that the process of putting water on the surface and rubbing it until it lathers creates a huge variable. How much or how little soap is dispersed in the water can affect the reading of the pH. None of the instructions detailed creating a definitive solution containing a specific ratio of soap to water. Some of the tutorials even negated specifying what kind of water to use! (As we mentioned earlier, tap water can vary a lot and throw off the readings.)
If pH strips are all you've got and you are eager to pH test your soap, using a specific solution and higher quality pH strips will help get some of these variables under control. (We'll talk about how to do that in a minute!) That being said, using pH strips is not the most ideal method of checking a soap's pH.
Using Phenolphthalein Drops to Test Soap pH
Phenolphthalein drops are most commonly recommended for testing liquid soap and hot process soap during the cook to identify whether the soap is "done" or if the soap needs to be neutralized. And while phenolphthalein drops can kind of serve that purpose, most of the tutorials and instructions I found directed you to place the drops directly on the soap. Pure soap, whether it's liquid soap paste or hot process soap, isn't an aqueous solution. And phenolphthalein drops, like other methods for pH testing soap, should be used in a solution.
 Phenolphthalein drops should be used in a solution rather than directly on soap, and can only tell you so much.
Phenolphthalein drops should be used in a solution rather than directly on soap, and can only tell you so much.
The main issue with phenolphthalein drops is how they work as a pH indicator. When added to a solution, phenolphthalein changes from clear to pink, depending on the pH of the solution. The initial color change from colorless to pale pink happens at a pH of 8.2, and deepens to a dark fuschia as the pH rises to 9.8. If used in a solution with a higher pH, they remain bright deep pink.
 Phenolphthalein drops indicate a solution's pH by changing from a colorless liquid to a deep pink. The color indication ranges from a pH of 8.2 to 9.8, and does not change outside of that range.
Phenolphthalein drops indicate a solution's pH by changing from a colorless liquid to a deep pink. The color indication ranges from a pH of 8.2 to 9.8, and does not change outside of that range.
This color change can be useful if you are wanting to bring the pH of a product below 8.2, which often requires the use of weak organic acid, such as citric acid. It can also be useful to titrate a soap solution to measure alkalinity, which is the case in common instructions for neutralizing liquid soaps.
However, pure soaps as a general rule have an alkaline pH, ranging from 8 to 10, so phenolphthalein drops don't necessarily indicate whether a soap is done cooking or safe for use. A liquid soap with a pH of 8.1 and a liquid soap with a pH of 8.3 (which crosses the color indication line for phenolphthalein drops) isn't going to perform wildly different as a basic cleanser. Plus, a soap with a pH of 10 (which is normal) will test exactly the same as a soap with a pH of 13 (which is not normal.)
How to pH Test Handmade Soap
Now that we've talked about all the issues with pH testing soap, let's get down to business with how to pH test soap in the most ideal way.
To do this, let's turn to a source for professional standards, ASTM International (American Society for Testing and Materials International). They are an international organization that develop and publish technical standards for a wide range of materials, products, systems, and services. And luck would have it that they have a standard for exactly what we need: ASTM D1172 - 15 (Standard Guide for pH of Aqueous Solutions of Soaps and Detergents).
Now, I would guess that most of us do not have the fancy equipment that the standard dictates for pH testing soap. (It recommends using a Fischer Accuphast combination electrode or Orion Ross Sure Flow electrode.) However, it is possible to follow the standards in procedure relatively closely!

To put this to work, I'm sharing pH testing results using a variety of methods/pH testers on a bar of soap that is three days old (relatively fresh, but should be mostly saponified at this point). The bar of soap is from the middle of the loaf, and partially gelled. There are no obvious signs of separation or excess alkali.
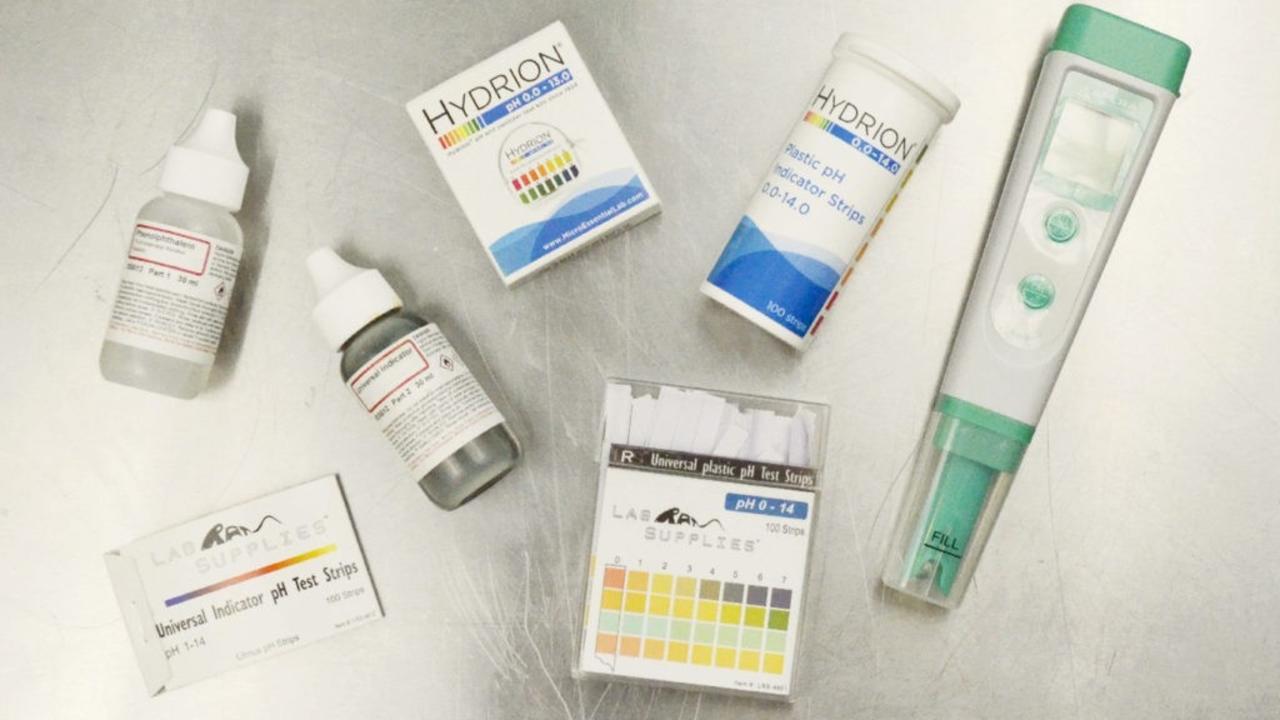
Here's the list of pH testing materials:
- Hydrion pH Paper with Dispenser and Color Chart - Full Range InstaChek (pH 0 to 13)
- Hydrion Spectral Plastic pH Strips (pH 0.0 to 14.0)
- Lab Rat Supplies Universal Indicator pH Paper Test Strips (pH 1 to 14)
- Lab Rat Supplies Universal Plastic pH Test Strips (pH 0 to 14)
- 1% Phenolphthalein Solution
- Universal pH Indicator Solution (Comes in a kit with phenolphthalein and bromothymol blue, which I wanted for homeschooling!)
- Apera Instruments AI209 PH20 Waterproof pH Tester
The first steps described in the standard is preparing the reagent, listing distilled water or equivalent, by removing the carbon dioxide by boiling or purging with carbon dioxide free air. The standard instructs to protect the distilled water with soda-lime or soda-asbestos while cooling and in storage. The next steps in the standard explain making a soap solution, and bringing it to an accurate temperature for pH testing.
My goal here is to follow the standard as closely as possible while still being practical for a handmade soapmaker, so let's dive in:
First, I boiled my distilled water, which will serve two purposes: help eliminate carbon dioxide and help dissolve the soap. Next, I shaved pieces of the soap from the side of the bar, from top to bottom, using a paring knife, into a clean disposable cup.
I'm aiming for a 1% soap solution, meaning 1% of the solution is soap and 99% of the solution is distilled water. To make it easy, I weighed approximately 1 gram of soap and 99 grams of distilled water on my American Weigh Scale (AWS-100). (I use this scale more often for blending essential oils and other tiny tasks, it's pretty handy and affordable!)
 Weigh 1 gram of soap for pH testing on a gram scale.
Weigh 1 gram of soap for pH testing on a gram scale.
Next, I added the distilled water, with a temperature reading of about 70° C (158° F), to a glass mason jar and then added the soap shavings. (The distilled water is still hot from boiling! As I mentioned, it was boiled directly beforehand.) Using a mini-whisk, I stirred the solution until the soap dissolved.
 A prepared 1% soap solution ready for pH testing!
A prepared 1% soap solution ready for pH testing!
The standards mention that you should conduct the pH test at 40° C (plus or minus 2° C), which is about 104° F and that the solution should be rapidly cooled. To do so, I placed my mason jar is an ice bath and kept an eye on the temperature while I prepared the pH testing materials.
Each one of the pH testing materials I used came with varying levels of instructions for accurate readings, so I defaulted to each individual one for instructions. For instance, some pH testing strips say to submerge the strip for 30 seconds while others say to "dip the strip" in the solution.
Here's the results of pH testing my soap with various methods:
 The Hydrion Paper Strips were obviously inaccurate, as we know that cold process soap cannot naturally test at a pH of 7.
The Hydrion Paper Strips were obviously inaccurate, as we know that cold process soap cannot naturally test at a pH of 7.
 The Hydrion Plastic Strips were not any better (typically, plastic strips are little bit better). It was impossible to determine if these strips were indicating a pH of 5 or 6, but we know that neither is accurate.
The Hydrion Plastic Strips were not any better (typically, plastic strips are little bit better). It was impossible to determine if these strips were indicating a pH of 5 or 6, but we know that neither is accurate.
 Lab Rat Supplies Paper Strips were realistically closer, reading a pH of 8. However, since this soap is fresh and contains the blend of oils that it does, it should test higher.
Lab Rat Supplies Paper Strips were realistically closer, reading a pH of 8. However, since this soap is fresh and contains the blend of oils that it does, it should test higher.
 The leader of the pack was found in Lab Rat Supplies Plastic Strips, which gave a pH reading of 9, which seems both plausible and realistic for this bar of handmade soap.
The leader of the pack was found in Lab Rat Supplies Plastic Strips, which gave a pH reading of 9, which seems both plausible and realistic for this bar of handmade soap.
 Even though phenolphthalein isn't very useful for pH testing, I did want to share what it would look like if used. The color of this photo is little off, but the solution definitely tested a relatively deep pink, as it should.
Even though phenolphthalein isn't very useful for pH testing, I did want to share what it would look like if used. The color of this photo is little off, but the solution definitely tested a relatively deep pink, as it should.
 Next, I wanted to try using an Universal Indicator solution to test handmade soap. Like the other pH testing methods, this indicator has a range of pH 1 to 14, but the color shift can sometimes be hard to differentiate.
Next, I wanted to try using an Universal Indicator solution to test handmade soap. Like the other pH testing methods, this indicator has a range of pH 1 to 14, but the color shift can sometimes be hard to differentiate.
 The 1% soap solution tested as a pH of 10 using the Universal Indicator, which is right around where it should be. If you want to try a fun science experiment with your kidlets, red cabbage juice is a universal pH indicator, too!
The 1% soap solution tested as a pH of 10 using the Universal Indicator, which is right around where it should be. If you want to try a fun science experiment with your kidlets, red cabbage juice is a universal pH indicator, too!
 The Apera pH Meter tested where I expect as well, with a reading of 9.6 to 9.9, depending on the temperature. This particular meter supposedly temperature compensates, so I wanted to test that and it seems to do a pretty good job.
The Apera pH Meter tested where I expect as well, with a reading of 9.6 to 9.9, depending on the temperature. This particular meter supposedly temperature compensates, so I wanted to test that and it seems to do a pretty good job.
Alright, so pH testing soap is all sorts of crazy...
After all this research and work, I have a few key takeaways about pH testing soap, whether it's cold process, hot process, or liquid soap. Here's the quick hit list:
- pH strips are terrible. Most of the pH strips I have used (both for this article and in the past) have been wildly inaccurate. The good news is that lab-quality plastic strips don't seem to do too bad off a job, if your curiosity is killing you.
- pH testing soap is largely inaccurate. Bar soap, liquid soap paste, and hot process soap are still just soap, and even when you make a soap solution, you are creating a colloid (where tiny little soap molecules are suspended in water). Since pH testing relies on an aqueous solution, it's difficult to get an accurate reading regardless of how you do it.
- If you want to pH test your soap, some methods are more accurate than others. Both the universal indicator and the inexpensive pH meter did a decent job of giving a pH reading, especially when compared to the wide range of readings from pH strips. If I had to choose one method of all the ones I've tested, I'd stick to the pH meter (especially as it would be useful in lotion making too).
- No matter the method, pH testing soap isn't as useful as many soapmakers think it is. Your soap's pH doesn't saying anything about how mild or gentle it is, since it's a wash-off product. While pH testing your soap can be helpful as a diagnostic tool in the troubleshooting process, it shouldn't be relied on as the sole method of identifying problems with a batch.
- Which brings us to the final takeaway: if your soap pH reads higher than it should, give it time. I've witnessed too many soapmakers throw away batches of soap (both liquid and bar soap) because their pH test came out with a 9, 10, or 11. Before you make any drastic moves, give the soap a little time to finish doing it's thing. And then pH test your soap again.
When it comes down to it, I know that soapmakers are going to pH test soap however they prefer (including that pesky tongue test!) However, I hope by working through the rundown on pH testing handmade soap, I've given you some food for thought and other options. Leave a comment down below, and let me know if you learned anything new or what you think!
Recommended Reading & Sources
- PubMed: Personal cleanser technology and clinical performance; by Syed Abbas, Jessica Weiss Goldberg and Michael Massaro (2004)
- PubMed: The long-term use of soap does not affect the pH-maintenance mechanism of human skin; by Takagi Y, Kaneda K, Miyaki M, Matsuo K, Kawada H, Hosokawa H (2015)
- PubMed: Effects of soap and detergents on skin surface pH, stratum corneum hydration and fat content in infants; by Gfatter R, Hackl P, Braun F (1997)
- PubMed: A comparative study of the effects on the skin of a classical bar soap and a syndet cleansing bar in normal use conditions and in the soap chamber test; by Barel AO, Lambrecht R, Clarys P, Morrison BM Jr, Paye M (2001)
- Influence of cleansing product type on several skin parameters after single use; by Mirela Moldovan and Alina Nanu (2010)
- The Effect of Detergent on Skin pH and Its Consequences; by Hans Christian Korting, Otto Braun-Falco
- Scientific Soapmaking: The Chemistry of the Cold Process by Kevin Dunn
- Kevin Dunn's website, which lists old lectures and white papers!
Want to snag weekly advice on building a successful soap biz directly in your inbox?
Of course you do! Sign up for our newsletter below for more tips and tricks to make bank in your biz.

